Safety is of primary concern when designing medical devices. After all, their purpose is to improve or maintain heath. Since standards such as the EN 60601-1 set protection guidelines based on the intended use of the medical device, compliance testing for safety depends on how the piece of equipment is classified. (Plastic syringes, EEG machines, and therapeutic X-ray machines require very different safety standards, for example.)
Is It a Medical Device with Electrical Connections?
To determine appropriate levels of protection, the standard first defines what items are covered. In the EN 60601-1 standard, electrical medical devices are defined as Medical Electrical Equipment. It may or may not be part of a Medical Electrical System. Any connected item necessary for normal use is considered part of the ME Equipment or ME System, which makes it a factor in meeting the safety requirements.
- Medical Electrical Equipment (ME Equipment) is electrical equipment that can transfer energy to or from a patient; has no more than one connection to a particular line power (“supply mains”); and that its manufacturer intends to be used for diagnosing, treating, or monitoring a patient. This definition includes the accessories the manufacturer defines as necessary for normal use of the ME Equipment.
- Medical Electrical System (ME System) is a combination of equipment specified by its manufacturer where at least one of the items is ME Equipment interconnected by a function connection. (This is a connection, electrical or otherwise, used to transfer signals, data, power, or substance. A wall socket, however, is not considered a functional connection.)
ME Equipment are categorized in different ways, depending on their form, intended function, and potential risk to patients and device operators.
Is It a Part You Can Touch?
How safe people might be when touching part of the device depends largely on whether it might be conducting electricity or heat. EN 60601-1 calls for standard tests to determine whether an area is touchable. Those areas are further categorized by whether they can be touched or whether they can be touched and that is the intention.
- Accessible Parts are components in the ME Equipment that testing have shown to be touchable, other than an Applied Part (an enclosure panel, for example).
- Applied Parts are components in the device that testing have shown to be touchable and are intended, in normal use, to come in contact to perform their function. This could be the ME Equipment itself or a connected auxiliary part in the ME System (ECG leads, or ultrasound transducers, for example).
What Type of Applied Part?
EN 60601-1 further classifies Applied Parts based on the level of intended contact with patients. What are considered acceptable levels of protection varies depending on which type of Applied Part has been assign. This is to assure that there are two layers of protection from potential harm. There are three types of Applied Parts:
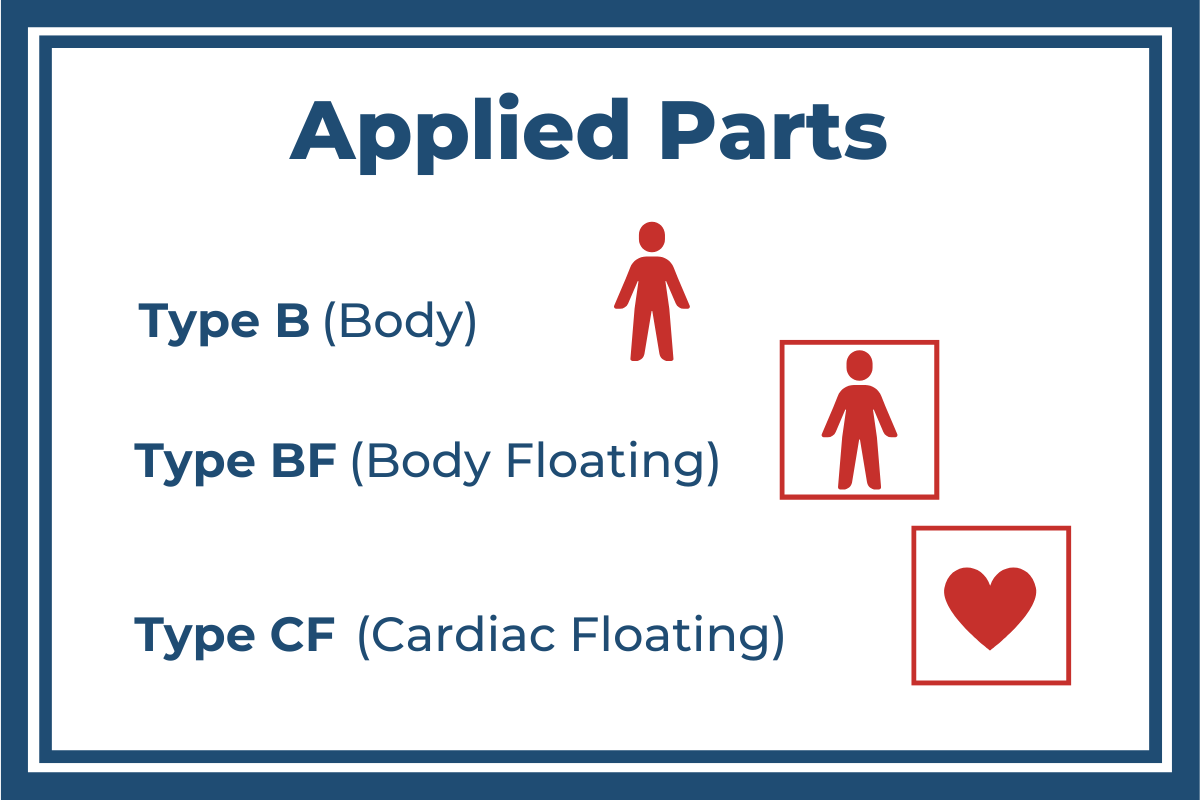
Type B Applied Parts
Of the three classifications Type B (Body) has the least potential risk and therefore the least stringent patient protection guidelines. It is used for Applied Parts that operate within a patient’s vicinity but are normally not conductive and not attached to the patient (hospital beds, MRI scanners, or X-ray machines, for example). In most cases, Type B Applied Parts are protectively earthed or separated from patients by at least one Means of Patient Protection (1 x MOPP).
Other Applied Parts are defined as “Floating” types. They are ones that are intended to make direct electrical contact with patients (for either treatment or monitoring) and therefore require the equivalent of at least two Means of Patient Protection (2 x MOPP).
Type BF Applied Parts
The next-most stringent requirements are for applications using Type BF (Body Floating) Applied Parts. These are Applied Parts that make direct electrical contact with the patient, except not directly to the heart (blood-pressure monitors and ultrasound, for example). Type BF requires a minimum of 2 x MOPPs.
Type CF Applied Parts
The Type CF (Cardiac Floating) Applied Parts are ones that are intended to make direct conductive contact with the heart (like defibrillators). For that reason, it requires the most stringent protection measures, including at least 2 x MOPPs.
What Kinds of Protection?
Since Applied Parts are designed to be touch by patients and device operators, EN 60601-1 calls for protections from excessive heat and electrical shock. Because each of the Applied Part Types have different applications, they each have different protection requirements. Maximum levels are set for temperature and leakage current, and testing standards for dielectric strength, creepage distances, and air clearance.
Applied Parts and Medical Power Supplies
Applied Parts of a medical device are the parts that are intended to come in contact with patients during normal operation. Any components that are connected to patient-connected devices are held to the same leakage current safety standards as the medical device itself.
While power supplies would not be in direct contact, they might be part of a medical device that includes one or more Applied Parts. Since leakage current and waste heat are intrinsic to a power supply, it is important to use a medical-grade power supply that complies with EN 60601-1 standards.
________________________
RAM medical power supplies have 2 x MOPP protection and can be used in medical devices that include Type B, BF, or CF Applied Parts as part of their system. Contact us for details.