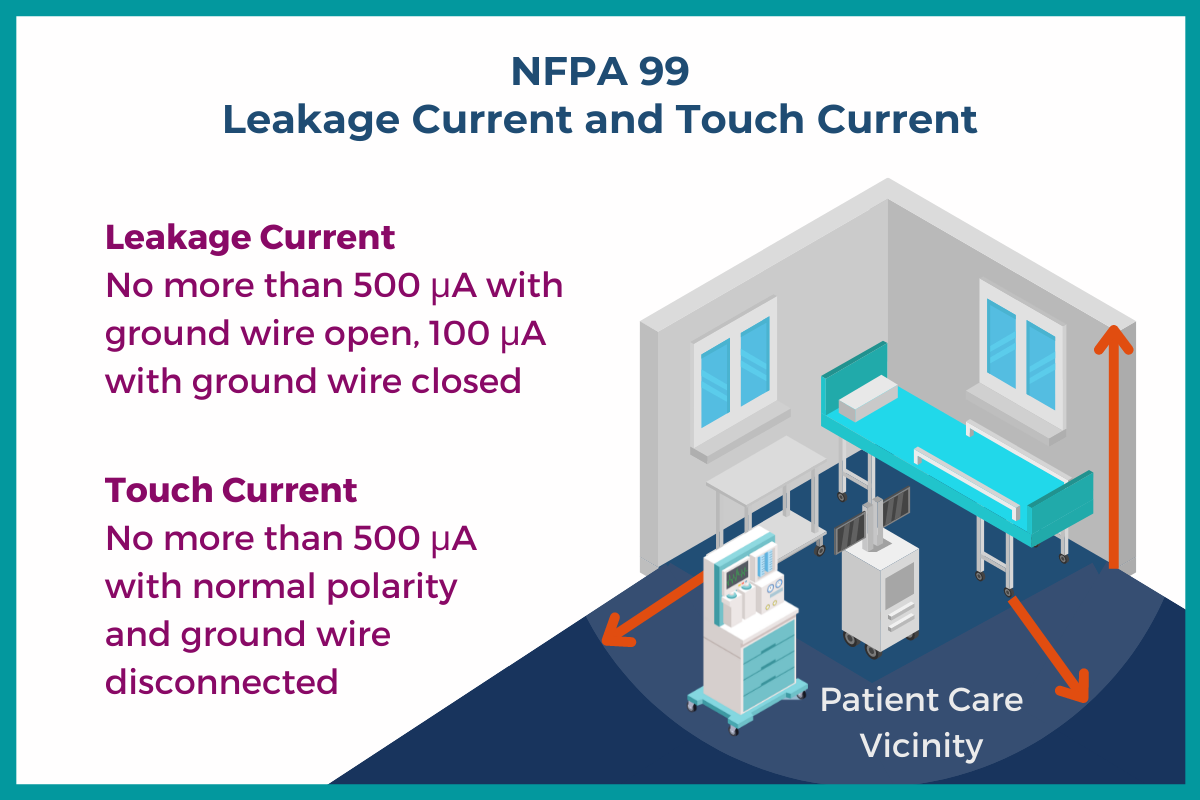
Low leakage current and touch current are critical in mitigating shock hazards around patients and caregivers. Like the widely used EN 60601-1 third edition, the NFPA 99 Health Care Facilities Code 2021 Edition from the National Fire Protection Association© sets safety standards regarding these hazards.
As might be expected, standards are stricter for equipment that patients might encounter. (What NFPA 99-2021 calls the Patient Care Vicinity.) While not a U.S. federal code, the NFPA 99 is widely adapted into code by many U.S. jurisdictions and widely regarded in other countries.
PC-Based Equipment and Medical Devices
It is very common to use PC-based equipment in conjunction with approved medical devices. When auxiliary equipment, like PCs, are connected to patient-connected devices, they are held to the same leakage current and touch current safety standards as the medical devices. If one connected piece of equipment is patient-connected, then all of the equipment must also meet the current levels set for patient-connected devices.
For example, a blood-pressure monitor is connected to a patient, and the monitor is also physically connected to a PC-type device to record the signals. Then, the PC is subject to the monitor’s leakage current and touch current requirements. The rationale is that auxiliary equipment may cause the patient-connected device to have fault currents beyond safe limits.
NFPA 99-2021 calls for complying with “the appropriate medical device or systems standards” and mentions EN 60601-1 in particular. Similar to EN 60601-1, NFPA 99-2021 states that leakage current “shall not exceed 100 μA for ground wire closed and 500 μA for ground wire open.” Touch current “shall not exceed 500 μA with normal polarity and the ground wire disconnected (if a ground wire is provided).”
Ways to Meet Leakage and Touch Current Standards
You can meet the touch and leakage current safety requirements in medical devices and auxiliary PC-based equipment in two different ways:
- Use a medical-grade PC power supply,
- Use a medical-grade isolation transformer.
Isolation transformers can be bulky and heavy and tend to get damaged because they are mounted externally. There is also a slight risk that someone could unplug the device from the isolation transformer and plug it directly into a non-isolated outlet.
A much smaller, medically certified PC power supply built into the PC chassis is a much better solution.
For further information, please see:
What Are Patient Care Spaces in the NFPA 99 Code?
6 Things to Know About Leakage Current in Medical Devices
________________________
Choose RAM Technologies certified medical PC power supply to help you meet NFPA 99 requirements. Contact us to find out more.