Safety is a primary concern when designing healthcare facilities patient care spaces and the electrical medical devices used in them. Medical device designers may be aware of the safety requirements in the widely used EN 60601-1 standard. They should also be aware of the code specified by the National Fire Protection Association (NFPA), which is required in many locations.
The NFPA 99 Health Care Facilities Code 2021 covers many aspects of safety “to minimize the hazards of fire, explosion, and electricity to the patients, staff, or visitors in health care facilities.” This includes electrical equipment near patients.
Compared to the average person, a patient is less likely to be able to move away from hazard and more likely to need oxygen, which poses a fire risk. The NFPA 99-2021 code therefore calls for stricter safety standards near patients. Look at:
- Do you intend to use the product as a medical device?
- Will the medical device be used in a healthcare facility?
- Within the facility, is the area used for patient care?
- In the patient-care area, what is the risk of harm or discomfort?
- Is the device within reach of a patient?
Is it patient care-related electrical equipment?
In the NFPA 99-2021 code, Patient Care-Related Electrical Equipment are the electrical devices “intended to be used for diagnostics, therapeutic, or monitoring purposes” that are found within the Patient Care Vicinity. (In EN 60601-1 these are Electrical Medical Equipment.)
Will the medical device be used in a healthcare facility?
The NFPA provides safety recommendations for all types of buildings, but the NFPA 99 code covers healthcare facilities in particular. NFPA 99-2021 defines healthcare facilities as buildings, parts of a building, or mobile enclosures where “human medical, dental, psychiatric, nursing, obstetrical, or surgical care is provided.” Some examples are hospitals, nursing homes, mobile medical offices, and dental offices.
Is the medical device in a patient care space?
NFPA 99-2021 defines a Patient Care Space as any space in a healthcare facility where patients are intended to be treated or examined. This could be areas like examination or hospital rooms but not reception areas or corridors.
What is the risk of harm or discomfort if equipment should fail?
Patient care, and the spaces they occur in, could vary a lot in a hospital or other healthcare facility. NFPA 99-2021 categorizes different spaces based on how much risk to patients, staff, or visitors might occur if equipment or a system should fail. The higher the risk, the more precautions the code calls for.
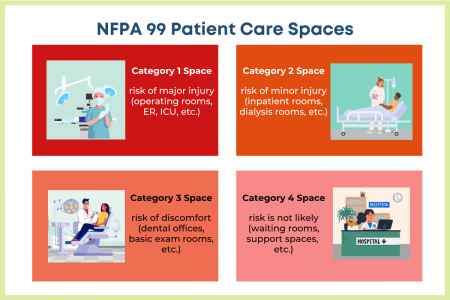
The Patient Care Spaces risk categories are:
- Category 1 Space: A space where “failure of equipment or a system is likely to cause major injury or death of patients, staff, or visitors.”
Examples: critical care spaces like operating rooms, emergency rooms, or the ICU. - Category 2 Space: A space where “failure of equipment or a system is likely to cause minor injury to patients, staff, or visitors.”
Examples: inpatient bedrooms or procedural rooms such as for dialysis. - Category 3 Space: A space where “failure of equipment or a system is not likely to cause injury to patients, staff, or visitors but can cause discomfort.”
Examples: basic exam and treatments rooms, such as a dental office. - Category 4 Space: A space where “failure of equipment is not likely to have a physical impact on patient care.”
Examples: waiting rooms and other support spaces.
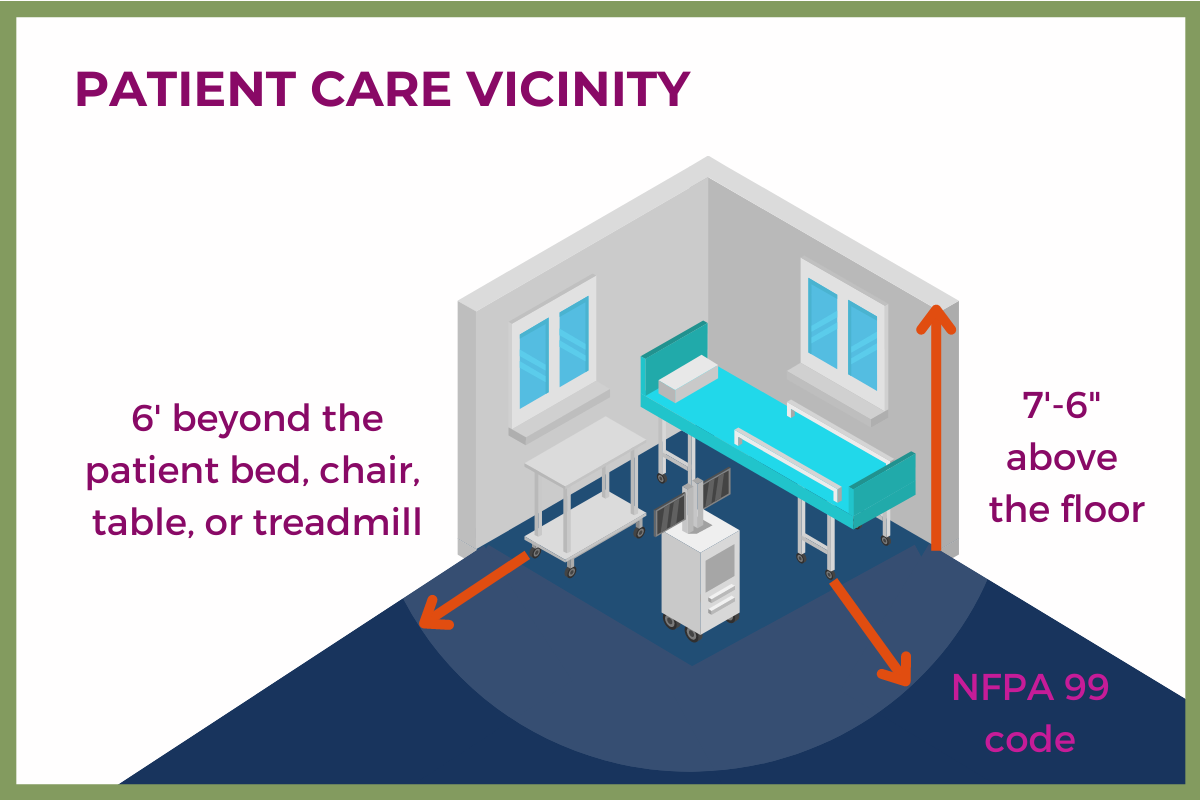
Is the equipment or system within the patient care vicinity?
The NFPA 99 Patient Care Vicinity is an area within the Patient Care Space “extending 1.8 m (6 ft) beyond the normal location of the bed, chair, table, treadmill, or other device that supports the patent during examination and treatment and extending vertically to 2.3 m (7 ft, 6 in) above the floor.”
Another words, the area generally within a person’s reach, where there is a much higher chance of contact with electrical equipment, sparks, therapeutic oxygen, or other potential hazards. Patient Care Vicinities call for the strictest requirements for electrical equipment in the area, both permanently connected and portable. (Much like the standards for Accessible Parts and Applied Parts in the EN 60601-1 standards.)
While not a federal code, the NFPA 99 is widely adapted into code by many U.S. jurisdictions and widely regarded in other countries. The best plan is to design your electrical medical device to comply with the NFPA 99 Health Care Facilities Code. No matter where your electrical medical device may be used in a healthcare facility, it will be compliant and, more importantly, designed to keep people safe.
________________________
If your PC-based equipment might be used in patient care vicinities, choose a RAM Technologies certified medical power supply to help you meet NFPA 99 requirements. Contact us to find out more.