In medical device design, safety is a priority. It’s the primary purpose of the widely used EN 6060-1 standard. EN 6060-1 is a body of “harmonized” safety standards regarding medical devices and systems that is recognized by the United States, Canada, EU, and other countries, based in part on the standards set by the International Electrotechnical Commission.
The level of safety requirements for medical devices set out in the standard is based on how close or electrically connected they are to people. Because patients tend to be more vulnerable than device operators, areas close to them have stricter requirements. It’s important to understand how patient areas are defined in the standard.
How are patient areas defined according to EN 60601-1?
EN 60601-1 divides healthcare spaces into “non-medically used” rooms (such as an office or storage room), “medically used” rooms (such as a treatment room), and the “patient environment” within the medically used room. Each area has increasingly more stringent safety standards.
What is Patient Environment?
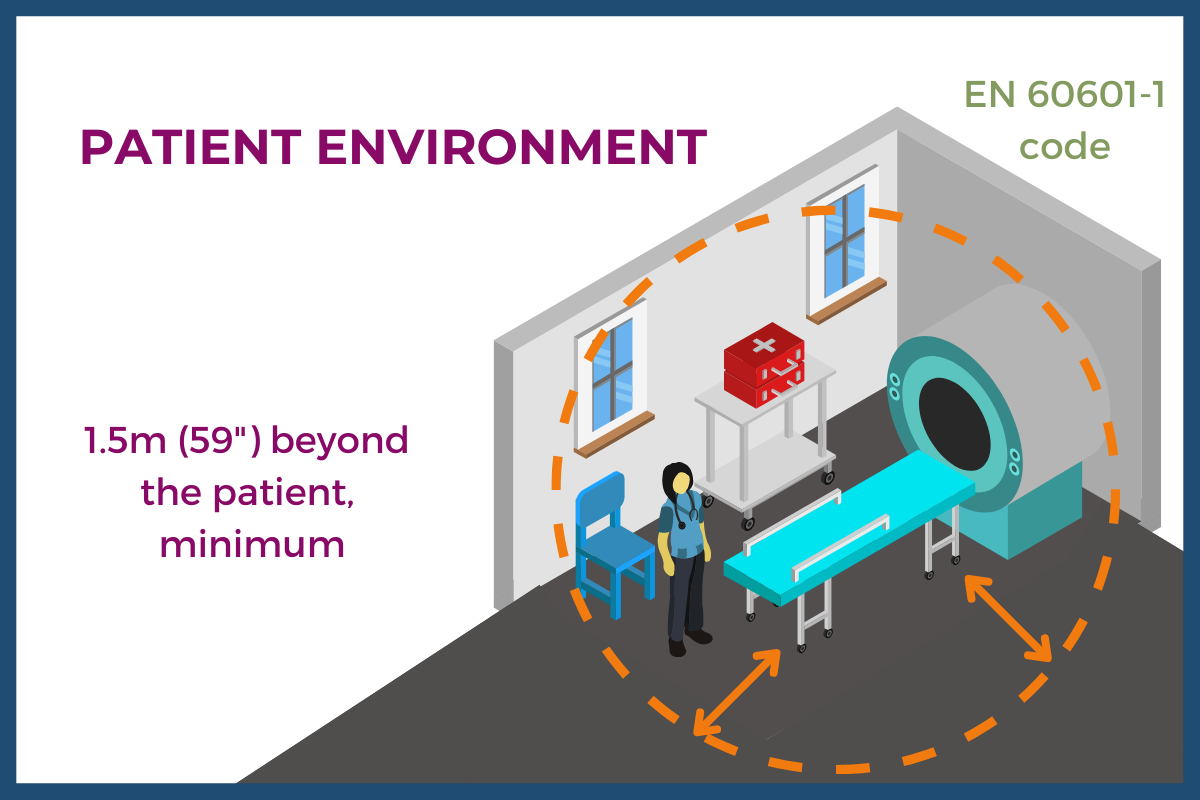
EN 60601-1 defines a Patient Environment as a volume “within a medically used room” around a patient. It is any area where “intentional or unintentional contact can occur” between a patient and either parts of a medical device or system or between a patient and some other person who is touching parts of the medical device or system. The standard calls for the “minimum extent” of the Patient Environment to be 1.5m (59”).
What is Medical Electrical Equipment?
Electrical medical devices are called “Medical Electrical Equipment” in the EN 60601-1 standard. When the medical device is part of a larger system, 60601-1 refers to that as a “Medical Electrical System.” In the standard they are usually shortened to “ME Equipment” and “ME System.”
There are additional safety standards for ME Equipment or ME Systems may be close to or touching patients (Accessible Parts and Applied Parts).
It is important to plan for the highest safety standards that your medical device might be subject to. That starts by understanding patient areas and their corresponding requirements.
________________________
If your PC-based equipment might be used in patient environments, choose a RAM Technologies certified medical power supply to help you meet EN 60601-1 requirements. Contact us to find out more.