If you want to sell medical devices in the United States, you must follow Federal Food and Drug Administration (FDA) guidelines, including properly classifying your product. Here are the steps:
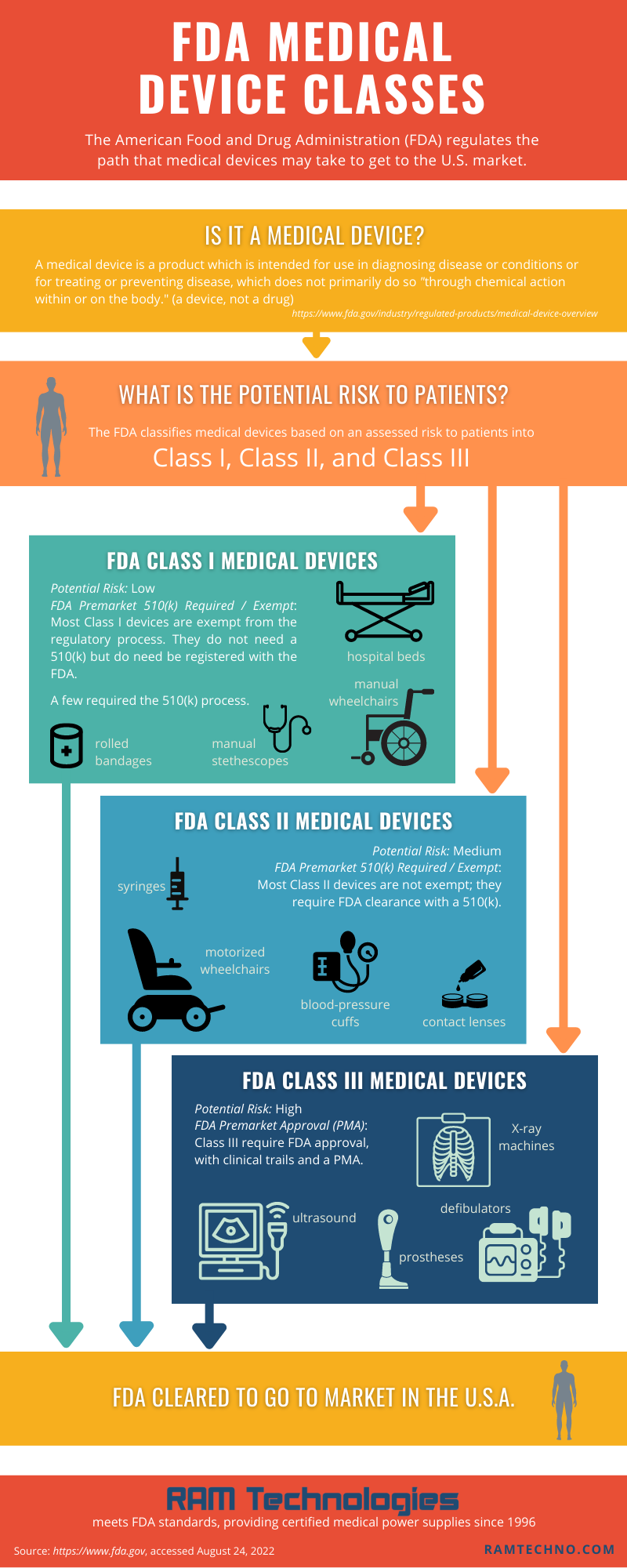
Is it a medical device?
The FDA defines a medical device as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is:
1. recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
2. intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
3. intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”
What FDA classification does it fall into?
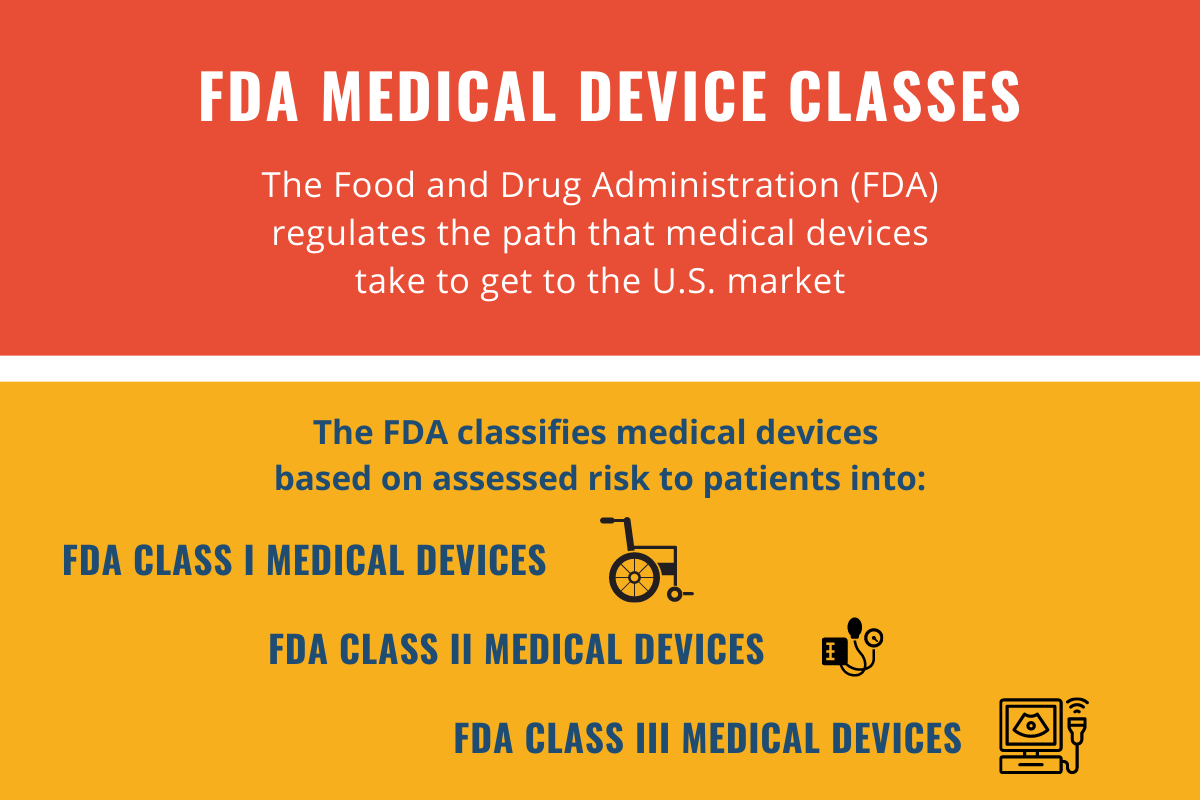
The FDA classifies medical devices based on an assessed potential risk to patients. The three FDA classes are: Class I, Class II, and Class III.
FDA Class I Medical Device
- Potential Risk to Patients: Low.
- FDA Premarket Notification 510(k): Most are exempt from the 510(k) process. Exempt products do not need a 510(k) but do need be registered with the FDA.
- Examples: Manual stethoscopes, elastic bandages, tongue depressors, hospital beds, non-electric wheelchairs.
FDA Class II Medical Device
- Potential Risk to Patients: Medium.
- FDA Premarket Notification 510(k): Most require FDA clearance with a 510(k), although some are exempt.
- Examples: Powered wheelchairs, contact lenses, handheld surgical instruments, infusion pumps, glucose tests.
FDA Class III Medical Device
- Potential Risk to Patients: High.
- FDA Premarket Notification 510(k): Class III devices present too much risk to be exempt. They require PMA.
- FDA Premarket Approval (PMA): Must submit clinical data and get premarket approval from the FDA.
- Examples: Implantable pacemakers, implants, cochlear implants, defibrillators, diagnostic tests.
FDA regulations are designed to bring products to the U.S. market that are safe and effective. Since there is a wide range of medical devices, with a wide range of risk factors, the amount of time and money required to get FDA clearance can also vary widely. Some devices are considered low risk because similar devices have already proven to be low risk. Others need clinic trials and FDA approval to demonstrate acceptable safety levels. When you understand which FDA Class your device falls into, you have a much better idea of what you must do before you can sell your device in the U.S. market, including design controls, standards to meet, and the estimated time and money needed to get FDA approval.
________________________
RAM Technologies has been providing certified medical power supplies for Class II and Class III devices since 1996. Contact us for details.